research

Tufts CSDD Impact report: Decentralized and hybrid trials deliver greater ROI than traditional trials
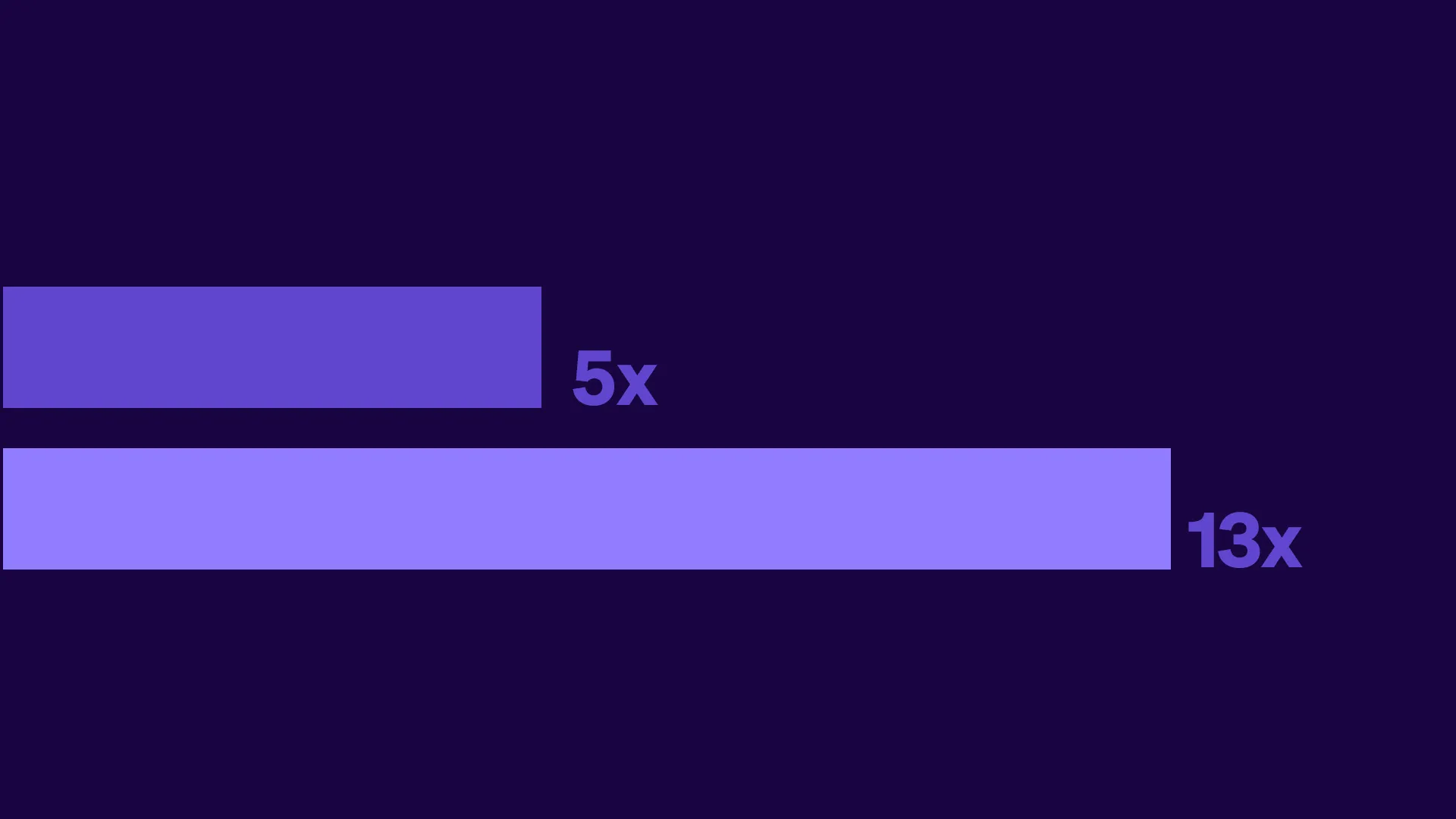
The Tufts Center for the Study of Drug Development completed an analysis of its financial modeling study of decentralized clinical trials using real data from Medable-enabled studies. These findings net financial benefits ranging from 5x for Phase II and 13x for Phase III trials, equating to roughly $10 million ROI and $39 million ROI, respectively.

Assessing the financial value of decentralized clinical trials
Deployment of remote and virtual clinical trial methods and technologies, referred to collectively as decentralized clinical trials (DCTs), represents a profound shift in clinical trial practice. To our knowledge, a comprehensive assessment of the financial net benefits of DCTs has not been conducted



.webp)
