
Challenge fuels change.
The Medable of today outpaces industry benchmarks and delivers game-changing results, fueled by a culture that thrives on progress and perseverance. As the industry shifts, we’re leading the way, not just as a product innovator, but as a partner invested in your success.
Delivery
100%
On-time go-live
Delivery
100%
On-time data transfers
Quality
90%
Patient eCOA adherence
Delivery
99%
Devices shipped on time
Help desk support
92%
First contact resolution
Delivery
5x
Faster study configuration
Delivery
35x
Faster first time eCOA creation than manual (no-code) build
Global access
120+
locales
“So proud of our collaboration which is positively impacting this space of the industry.”
GSK
Better today, ready for tomorrow
Product Innovation
By launching Medable AI, eCOA+, and Studio, we’ve streamlined trial execution, cut timelines by 50%, and automated critical tasks, empowering customers to focus on delivering life-changing therapies.
Process Excellence
Our sharpened focus on operational excellence has transformed how we deliver. With 98% device success and 95% on-time launches, we’ve built a foundation of precision and reliability to hit critical trial milestones seamlessly.
Partnership Culture
Through deep collaboration and patient-centric initiatives, we continue to build a culture of trust, innovation, and impact, ensuring every stakeholder benefits from meaningful progress.
Expanding access to life changing treatments.
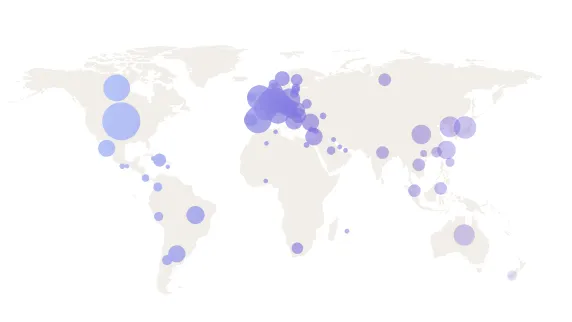
Expanding global access
Medable currently operates in 70+ countries, enabling digital and decentralized trials worldwide, including regions such as Japan, France, and underserved remote areas.
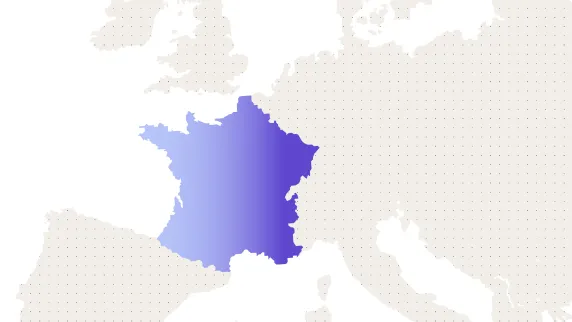
Regional expertise and regulation support
Medable became the first U.S.-based clinical trial platform provider to receive approval from France’s Commission Nationale de l’Informatique et des Libertés (CNIL) for its eConsent and eCOA solutions for a partner Sponsor company.

Expertise and patient input across diverse therapeutic areas
Medable currently enables digital trials across 26 therapeutic areas and works with our expansive Patient Caregiver Network, which includes 90+ patient advocates and caregivers across 12 countries, representing 67 diverse health conditions.
Experience the award-winning platform designed for sponsors, sites, and participants.




Driving progress for sites, patients, and the industry.
Together with our partners and customers, we look forward to shaping the future of clinical research one breakthrough at a time to continue enabling treatments to patients faster.


Medable’s 2024 was a year of exceeding key milestones and helping our customers reach new heights. Take a look at the difference Medable has made with our year-end impact report.
Related content


Driving a high-adherence LTFU trial without an EDC
Learn how Medable powered a decade-long, global long-term follow-up (LTFU) obesity study, achieving an impressive 97% patient retention rate without using a traditional EDC system all while delivering a compliant, scalable, and cost-efficient solution.


Eliminate clinical trial white space with the right AI strategy
It has become clear that our industry has reachedthe limits of human-only clinical development. As clinical trials have become increasingly complex, the endeavors that people alone can perform are no longer sufficient to generate the momentum needed to address the growing burden of human disease. This has led to longer drug development timelines and significant delays for patients. One large are of lost time is “white space,” definied simply as unproductive time caused by manual, sequential processes and fragmented data systems. Thankfully, a solution lies in agentic AI and its abilities to perform series of tasks.


Roadmap to adopting AI agents
The successful integration of AI agents in enterprise operations requires a balanced, deliberate approach. Drawing from recent research in Strategic Integration (SI) and agentic AI adoption within large enterprises, the following best practices help maximize value, manage change effectively, and mitigate common pitfalls. Medable Agent Studio specifically streamlines this process by providing robust tools, no-code simplicity, and built-in compliance and security standards.

.webp)

