Your co-pilot for clinical monitoring
Medable’s CRA Agent is an AI-driven solution that automates and optimizes clinical trial monitoring by proactively identifying and prioritizing site risks, generating comprehensive pre-visit summaries, and providing actionable recommendations to enhance trial oversight and compliance.

We estimate that CRA agents can take on up to 90% of the tactical and administrative work the CRA handles on a daily basis — from sending site-specific emails and reminders to tracking responses and updating systems. That means we can focus our time on the strategic decisions that move trials forward, while standardizing and streamlining the tasks that used to consume most of our day.

Connected monitoring across systems
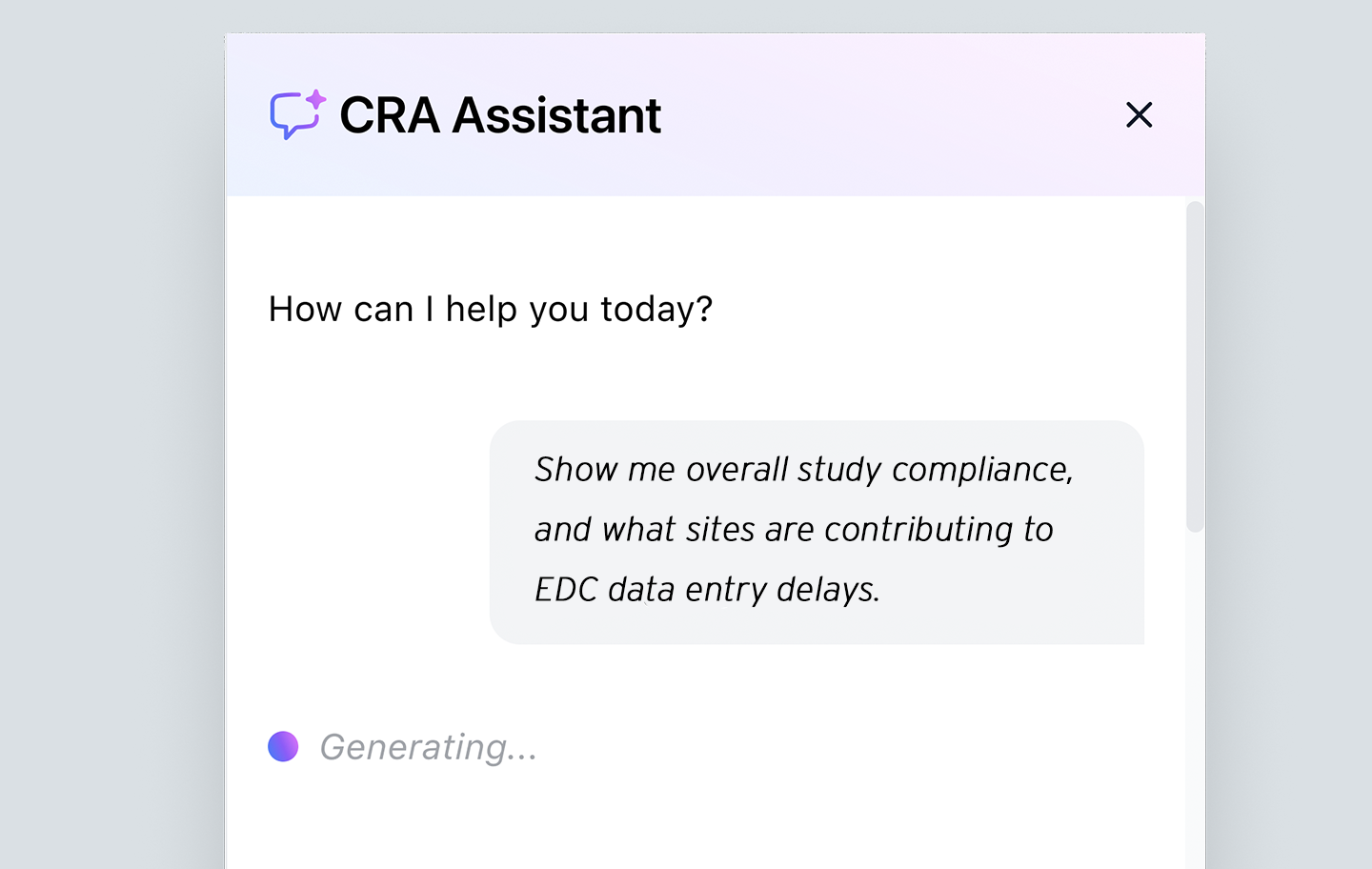
Perceive signals across the entire clinical data ecosystem by unifying CTMS, RTSM, EDC, labs, consent, and safety into one view. Turn manual checks into automated insights, instantly highlighting which sites are on track and where intervention is needed, saving valuable time, system-hopping, and manual data compilation.
Human-in-the-loop oversight
Easily identify risks and recommendations for best-next actions tied to your protocol, consent, and regulatory requirements. Stay assured with a transparent reasoning trail thatshows why each step is advised, enabling CRAs to collaborate confidently with sites, improve decision-making, enhance their compliance, all while freeing CRAs to focus on engagement and site-specific challenges.
Automated administrative tasks
Take advantage of automated routine tasks like drafting queries, sending emails, and updating CTMS/eTMF — always with human CRA oversight. Close the loop between planning and execution with real-time tracking that reduces administrative burden, ensures data consistency, and accelerates trial progress.
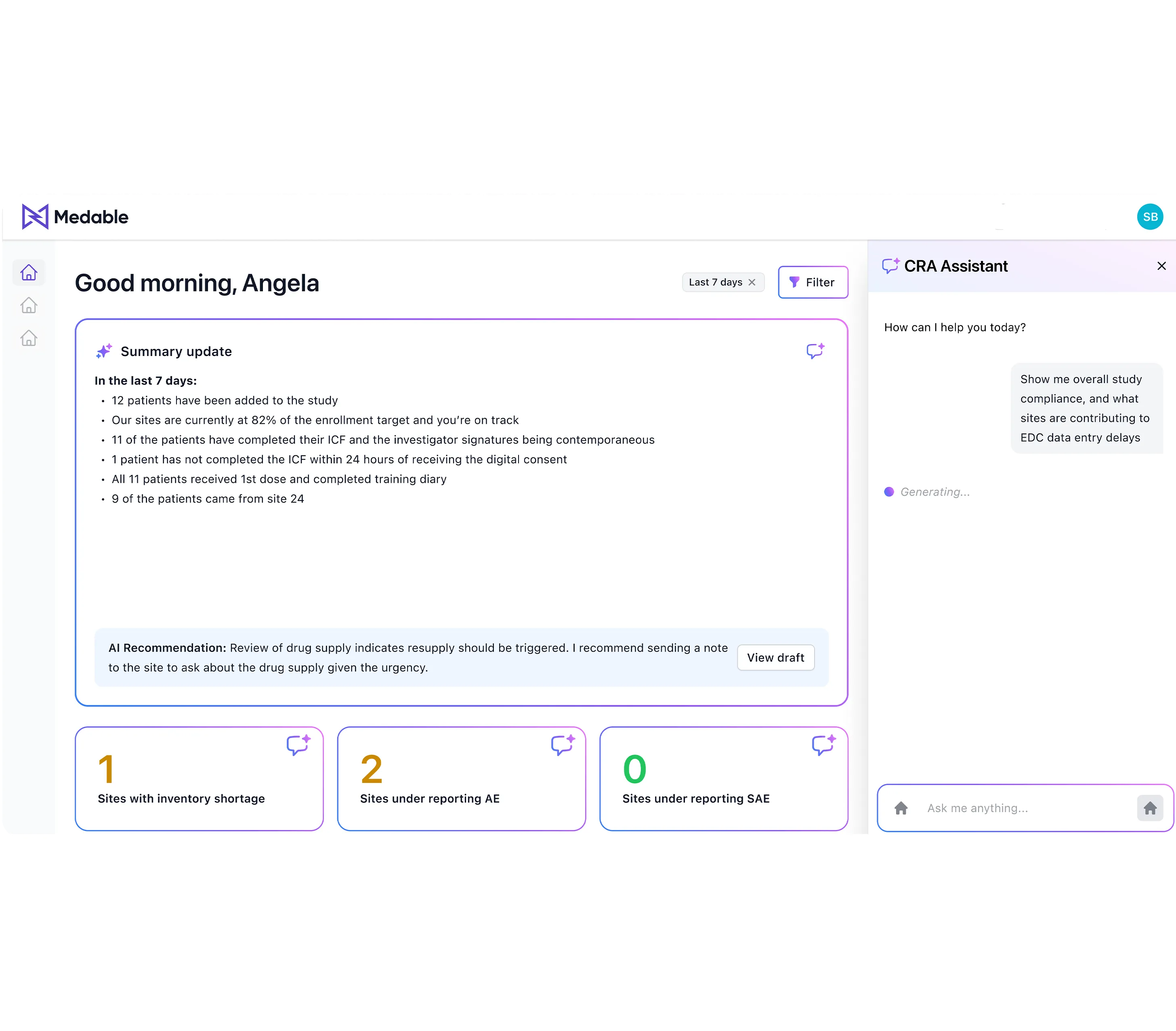
The latest from Knowledge Centers


Driving a high-adherence LTFU trial without an EDC
Learn how Medable powered a decade-long, global long-term follow-up (LTFU) obesity study, achieving an impressive 97% patient retention rate without using a traditional EDC system all while delivering a compliant, scalable, and cost-efficient solution.


Faster Trials, Programmatic Scale: Standardizing a Digital Approach Across Therapeutic Areas
Explore how AI and standardization are transforming clinical trial efficiency across multiple therapeutic areas in this expert-led webinar.


Building blocks: The ultimate guide to AI in clinical trials
Explore how artificial intelligence (AI) is fundamentally transforming every facet of clinical trials, from initial protocol design and patient recruitment to data management and regulatory approval. This comprehensive guide provides an authoritative, in-depth look at AI's role in accelerating drug development and improving patient outcomes, with special focus on emerging agentic AI technologies.

Eliminate clinical trial white space with the right AI strategy
It has become clear that our industry has reachedthe limits of human-only clinical development. As clinical trials have become increasingly complex, the endeavors that people alone can perform are no longer sufficient to generate the momentum needed to address the growing burden of human disease. This has led to longer drug development timelines and significant delays for patients. One large are of lost time is “white space,” definied simply as unproductive time caused by manual, sequential processes and fragmented data systems. Thankfully, a solution lies in agentic AI and its abilities to perform series of tasks.


.webp)
