Representation of Asian participants increased by six percentage points; American Indian/Alaska Native representation quadrupled; Female representation rose from 49.0% to 55.7%
PALO ALTO, Calif. – January 28, 2025 – Medable Inc., a leading provider of clinical development technology, today announced new data from the Tufts Center for the Study of Drug Development (CSDD)’s PACT Consortium linking decentralized clinical trial (DCT) approaches to notably higher proportional representation among select demographic subgroups. The new research suggests that more strategic implementation of DCT approaches in conjunction with more rigorous planning may meaningfully improve diversity in clinical trials.
The analysis of 69 clinical trials showed that 20.9% of participants identified as Asian when DCT components were deployed compared to only 14.2% when no solution was used. Proportional representation of indigenous communities (i.e., American Indian and Alaska Native) was nearly quadruple (1.9% versus 0.5%). Participation of women in DCT-enabled clinical trials was also significantly higher – 55.7% vs. 49.0%. However, the proportional representation of patients who identified as Black or of African descent in DCT-enabled clinical trials was not significantly different when compared to trials where no DCT support was provided. See Figure below.
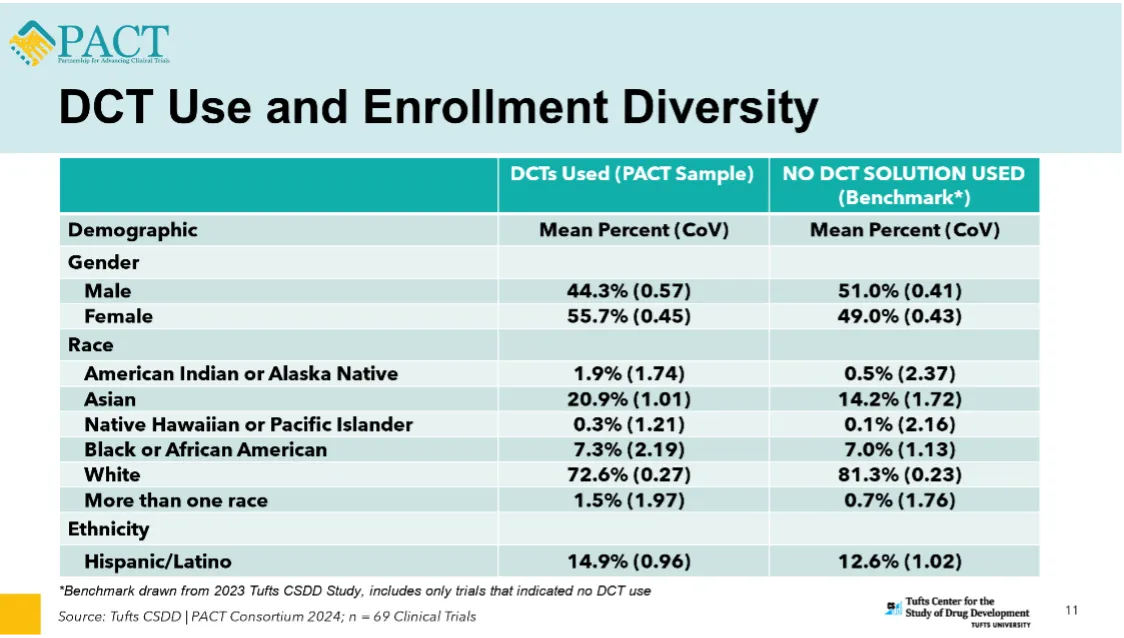
“The results show that DCT component use in clinical trials is associated with improvements in proportional representation by race and ethnicity but there is still wide variation in patient preferences, by disease condition, and by the type of DCT component deployed,” said Ken Getz, Tufts CSDD Executive Director and research professor. “Improvement in enrollment diversity and clinical trial access requires a very intentional and strategic approach. In a separate study, certain decentralized elements, such as local labs, were associated with significantly higher proportional representation among Black participants. As we collect more empirical evidence, it is very clear that one size does not fit all. Each clinical trial requires a custom approach driven by patient needs and preferences.”
The PACT Consortium – managed by Tufts CSDD and backed by over 30 top‐tier pharmaceutical organizations and technology providers including Medable – seeks to provide the industry with a data‐driven blueprint for action. AbbVie, Amgen, Gilead, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, GSK, Janssen, Novartis, Pfizer, Roche Genentech, and Sanofi contribute financial resources and frontline clinical insights, while the NIH, FDA, and National Cancer Institute provide additional oversight and expertise.
“The first year we focused on creating consensus on definitions and gathering as many trials as possible – now, close to 70 – and hope to double that next year,” said Dr. Pamela Tenaerts, Medable Chief Scientific Officer, and a founding member of the PACT Consortium. “Medable is fiercely committed to investing in evidence-generation on the impact of decentralized elements on clinical trials so we can uncover the indisputably best ways to apply technology to make a significant impact on research. PACT members already want to add more variables to test – it’s like peeling an onion. Every time you pull back a layer, you find new dimensions to explore.”
Tenaerts will be available at the 2025 SCOPE event in Orlando, FL, booth #717, to discuss the PACT Consortium findings. Medable will also be presenting with Christina Fawcett, Director of Digital Health Delivery at GSK on Wednesday, Feb. 5 at 9:55am.
Medable has deployed its software-as-a-service platform in more than 300 decentralized and hybrid clinical trials in 70 countries, serving more than one million patients and research participants globally. Customers have achieved impressive results – including 90% eCOA adherence and 50% cost reductions. A Tufts CSDD study also shows that, on average, decentralized trials can achieve net financial benefits from five to 13 times for Phase II and Phase III trials, equating to roughly $10 million ROI and $39 million ROI for an investment on average of $500K in Phase II and $1.5M in Phase III trials, respectively. Recently, the company launched Medable AI and Medable Studio to automatically convert outcomes assessments into fully digital eCOAs in seconds – now accessible on Google Cloud Marketplace.
About Medable
Medable is on a mission to get effective therapies to people faster. Its digital clinical trials platform enhances speed, scale, and patient access in clinical research, accelerating medicines for thousands of conditions without treatment or cure. Awarded Best Digital Health Solution by the Galien Foundation, Medable’s platform has been deployed in nearly 400 trials in 70 countries and 120 languages, serving more than one million patients globally. Medable is a privately held, venture-backed company headquartered in Palo Alto, California, and was listed for the second year in a row on the Inc. 5000 in 2024.
# # #
Media Contact:
Lisa Barbadora, Barbadora INK for Medable
+1 (610) 420-3413
lbarbadora@barbadoraink.com / media@medable.com







