
Related articles


Six steps to help you choose the right clinical trial partner
“This year, we’ve decided to stick to paper. We know what we’re getting and we’ve always done it this way.”
That was the unwavering response from a clinical operations lead at a pioneering biotech when asked why they still relied on paper diaries for patient-reported outcomes.
Despite the growing complexity of global trials, the promise of real-time data, and the surge of digital capabilities available today, some organizations have held tight to a method of clinical trial conduct that’s increasingly as outclassed as it is outdated.
It’s well known that individuals and organizations believe that change can be daunting, stressful, and difficult, especially when the old way is familiar and entrenched. However, much like anything else, having a partner who can help guide you through the process is massively important. But, how do you find the right partner for your trials?
Before locking in a vendor, organizations must engage in a deliberate process to assess their needs, align stakeholders, and set the foundation for long-term success. This blog explores the critical steps sponsors should take before selecting a digital partner, using insights and frameworks drawn from Medable’s therapeutic area standards and industry best practices.
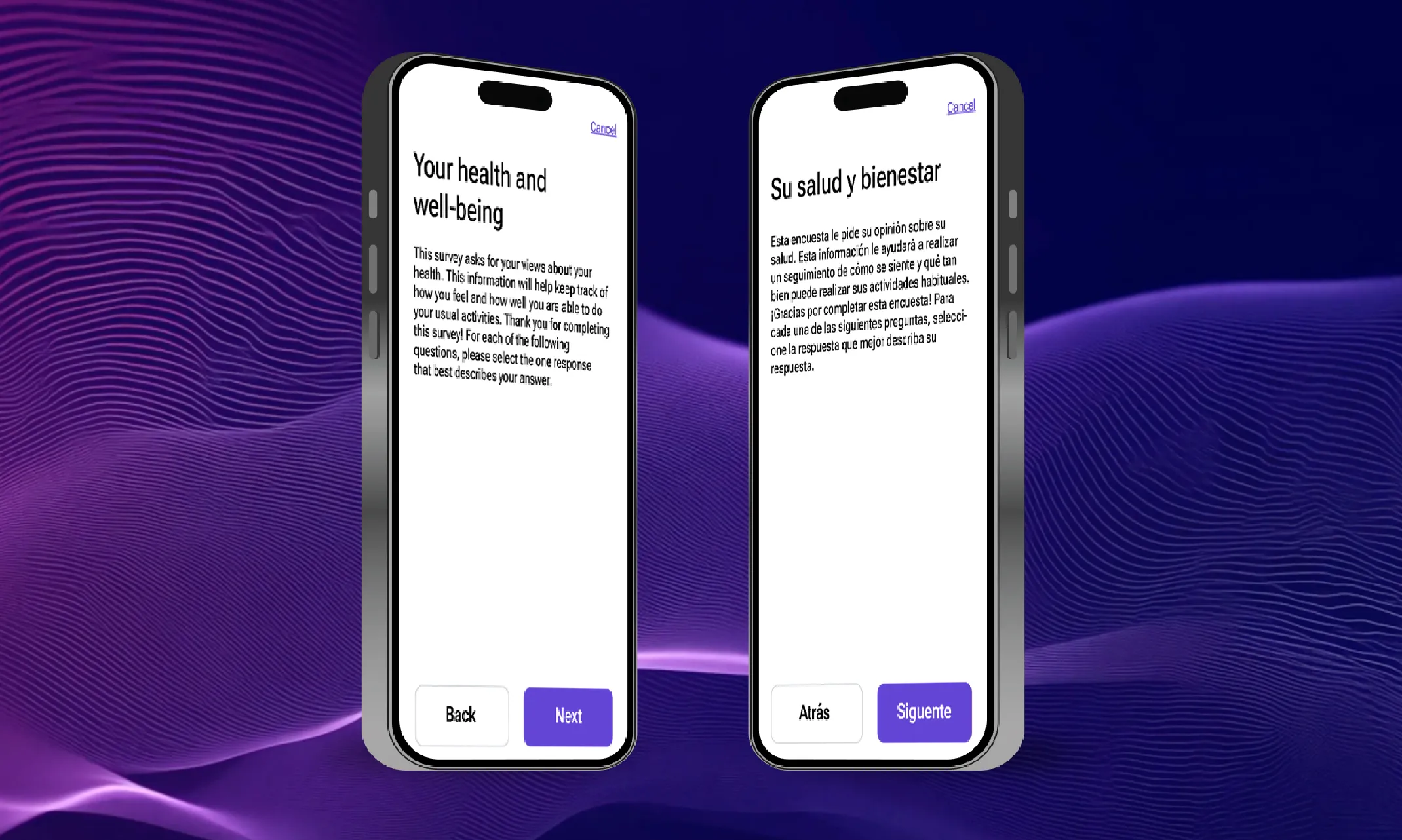

From bottlenecks to breakthroughs: How AI is transforming translation timelines
According to ClinicalTrials.gov, there are 3,046 multi-country trials being conducted this year. While many trials remain localized within a single country, there has been a definitive movement towards conducting trials in multiple countries, especially for larger, later-stage trials. This is driven by the positives that multi-country trials offer, like faster patient recruitment, lower costs in some regions, and the need for diverse patient populations.. However, behind the scenes, a critical bottleneck has been slowing many trials down. This bottleneck is the translation process that’s required to make trials work across multiple languages, locales, and regulatory bodies/organizations.
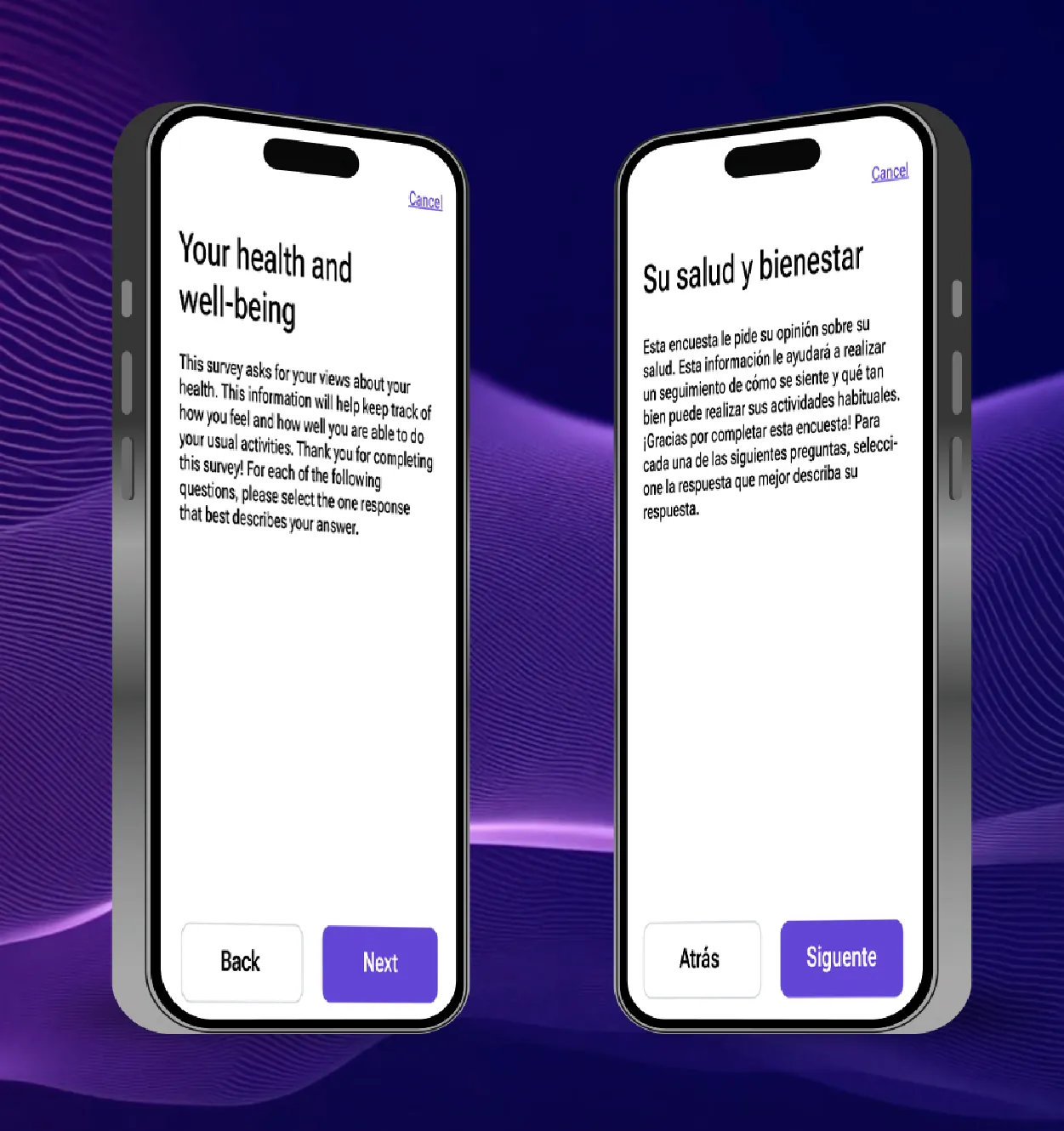

Case study: Removing translation bottlenecks with AI
Traditionally, translations and language migration create significant bottlenecks on the path to trial study go-live. The process is traditionally manual, linear, and resource intensive. To address these challenges, Medable partnered with Lionbridge, a leading translation services company to compare the status quo translation process against an AI-enabled approach powered by both companies’ proprietary new AI tools.


.webp)
